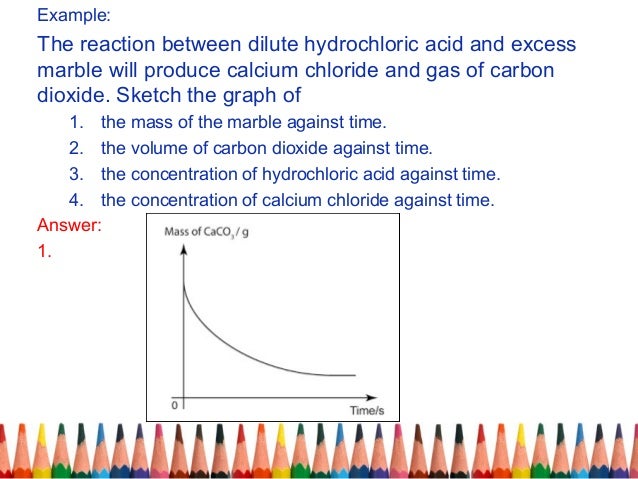
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Marble chips and hydrochloric acid graph.
There are many variables that affect.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
A stand to hold up the measuring cylinder.
The rate of this reaction can be changed by changing the size of the marble chips.
The variables that i shall be changing will be the concentration of hydrochloric acid and water.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
To investigate the effect of concentration on the reaction between marble chips and hydrochloric acid materials.
Conical flask glass jam jars measuring cylinder stop clock watch with seconds stop watch app direct reading balance dilute hydrochloric acid cotton wool marble chips method.
Place 40cm 3 of hydrochloric acid in an conical flask.
A conical flask contains the marble chips hydrochloric acid and the water that will make the reaction.