I will weigh out one gram of marble chips using a balance and put it in a conical flask and add to it a concentration of 50cm3 using water and hydrochloric acid.
Marble chips and hydrochloric acid experiment surface area method.
2 to investigate the effect of temperature of sodium thiosulphate in a reaction with hydrochloric acid.
An investigation of the reaction between marble chips and hydrochloric acid.
Finding the rate of reaction of marble chips and hydrochloric acid changing the surface area.
Changing factors independent variables.
To observe the effect of surface area particle size on the rate of reaction between marble chips calcium carbonate and hcl.
To investigate the effect of concentration of sodium thiosulphate in a reaction with hydrochloric acid.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
Finding the rate of reaction of marble chips and hydrochloric acid changing the surface area.
Add different sizes of marble chips solid crushed and powder concentration.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
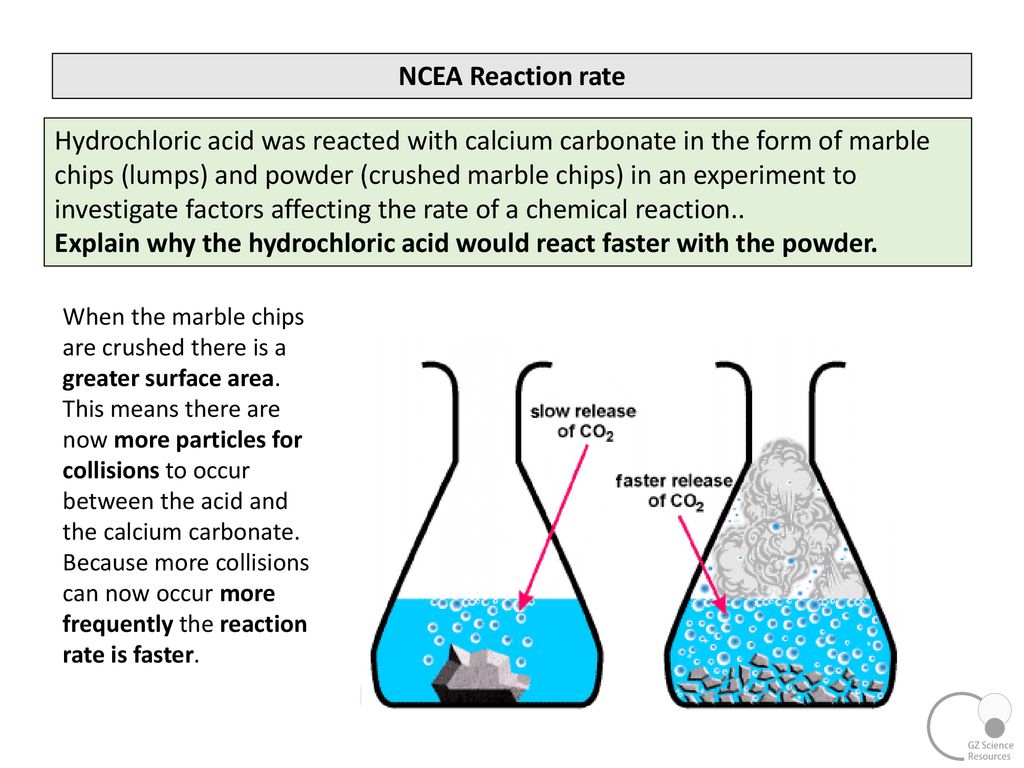
Smaller chips have a larger surface area.
Contains the marble chips hydrochloric acid and the water that will make the reaction.
There are many variables that affect.
Hcl calcium carbonate calcium chloride carbon dioxide water.
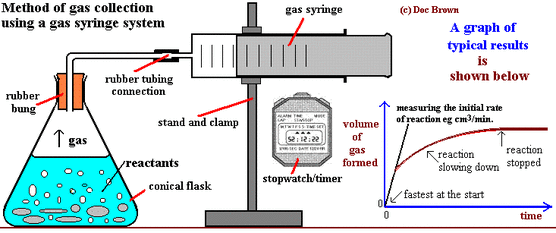
Use a capillary tube to connect this flask to a measuring cylinder upside down in a bucket of water downwards displacement.
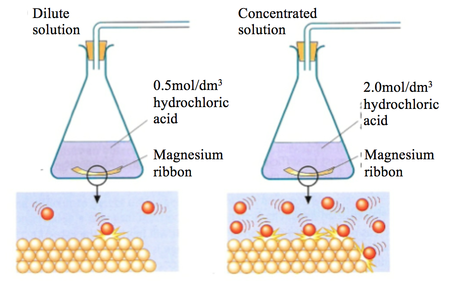
Add different concentrations of hydrochloric acid method.
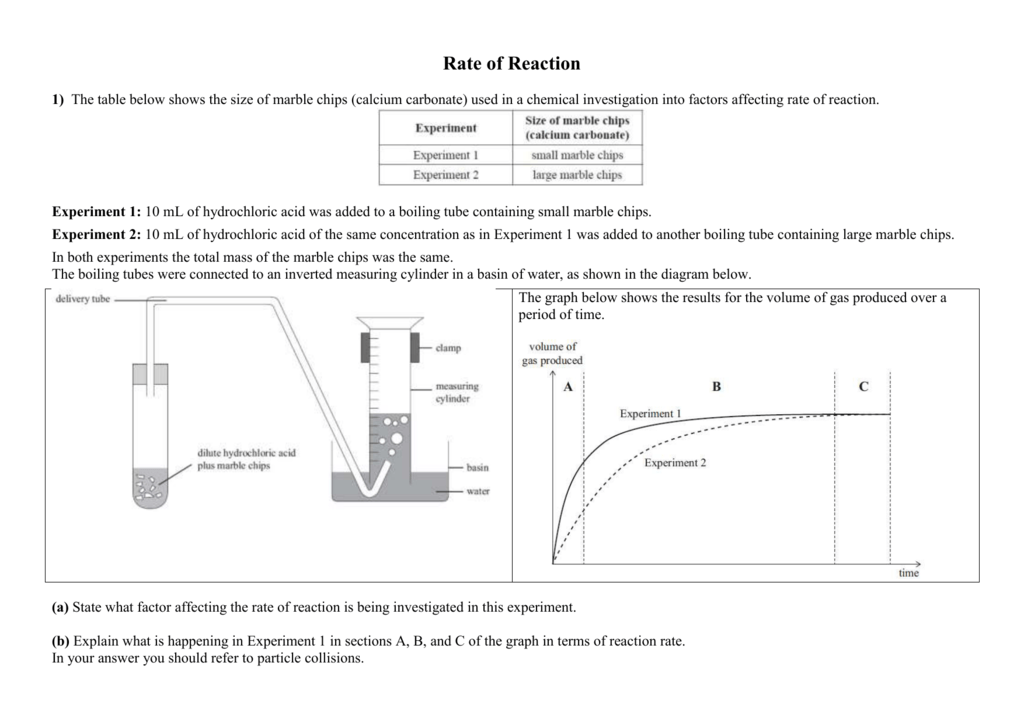
Equipment list the equipment i will be using for this experiment to see how the surface area of marble chips caco3 affect the rate of reaction when placed in hydrochloric acid are 25 ml of hydrochloric acid 3g of marble chips graduated cylinder a stop clock test tube 250 ml beaker delivery tube scale ceramic mount and a plastic dish.
Add hydrochloric acid into a conical flask.
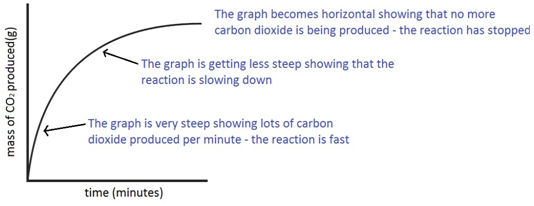
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
As the marble chips react with.
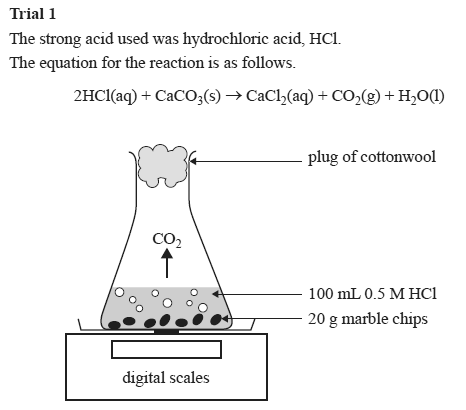
2hcl aq caco 3 s cacl 2 aq co 2 g h 2 o l the reaction rates of both large marble chips and small marble chips can be compared see below.
The surface area isn t always the same so even though the mass of the marble.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
And dilute hydrochloric acid calcium carbonate may be used in the form of marble chips.