In high concentrated acid the particles of the marble chip will move faster due to the more collisions between the particles of marble chips and the acid.
Marble chips and hydrochloric acid experiment method.
Measured 5ml of hydrochloric acid in the 10ml measuring cylinders and placed into each beaker separately.
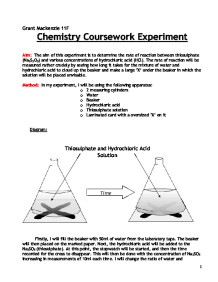
An outline of an experiment that could be used to find the time and hence rate of reaction of marble chips and hydrochloric acid.
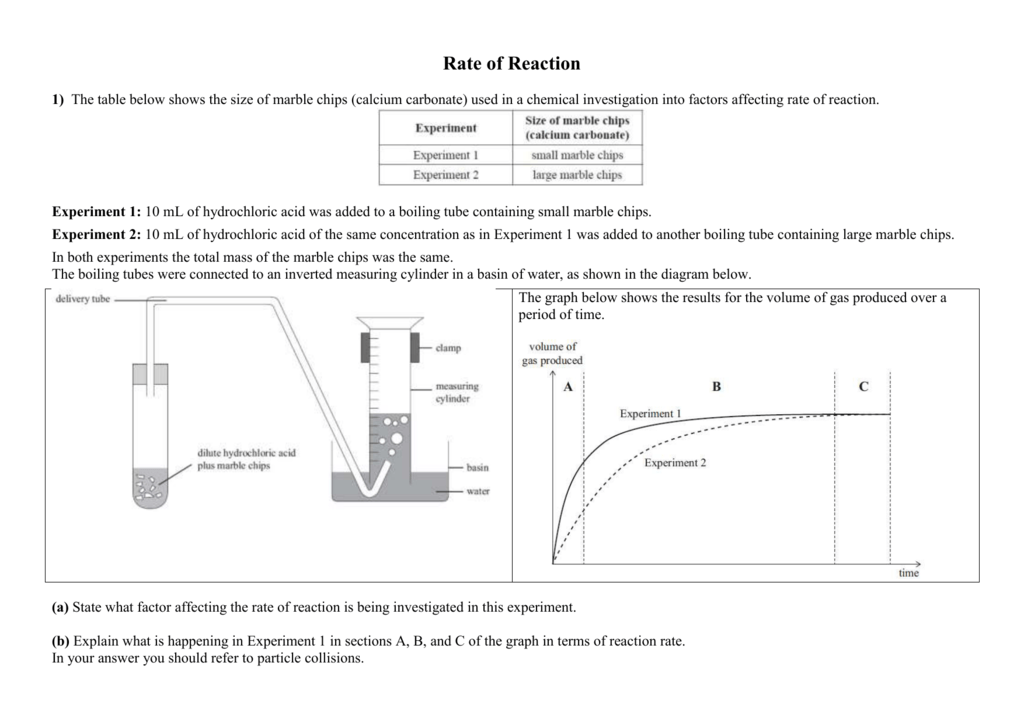
The rate of this reaction can be changed by changing the size of the marble chips.
I predict the higher the concentration of hydrochloric acid faster the reaction rate and more carbon dioxide will be produced as the time increases.
Marble chips placed onto pieces of paper.
Measured out 1ml of water in a 10ml measuring cylinder and placed into the test tube labelled 2.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
To start the reaction the flask is gently lent to one side causing the card to fall and the marble chips and acid to mix.
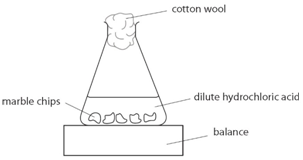
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
Plugged in scientific scales and weighed out 1g of marble chips for each test tube.
There are many variables that affect.
Marble is calcium carbonate and thus behaves in the same way.
Caco3 s 2hcl aq 61614.
Cacl2 aq h2o l co2 g in this experiment i am going to see if temperature affects the reaction rate between marble chips and hydrochloric acid by timing the release of carbon dioxide in the reaction.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Calcium chloride solution is also formed.
Marble chips and acid are placed in the flask but separated by a piece of card preventing the reaction from proceeding.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
I predict the higher the temperature the.
This apparatus is placed on a balance and the mass of the flask and its contents is read.